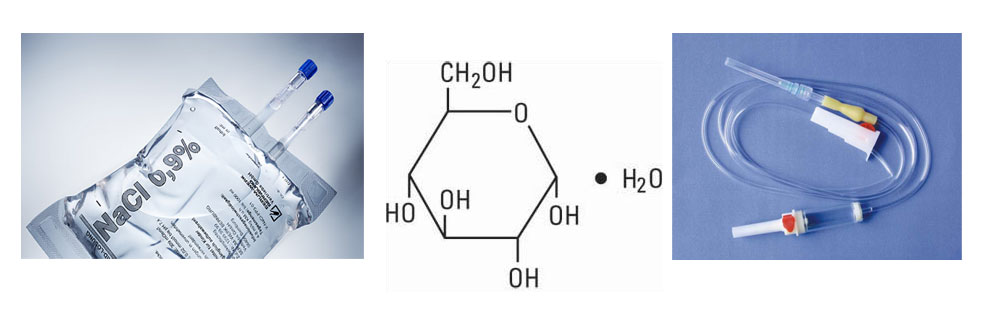
Products
Latest News
-
PSI Orion Infusion Limite ...
Date : 25, Nov 2023
... -
PRICE SENSITIVE INFORMATI ...
Date : 25, Nov 2023
... -
Proxy Form ...
Date : 28, Nov 2021
... -
PSI Q1 2021 ...
Date : 16, Nov 2021
... -
PSI and AGM, 2021 ...
Date : 14, Nov 2021
... -
Price Sensitive Informati ...
Date : 1, Jul 2021
ORION INFUSION LIMITED Registered Office:... -
AGM and EGM Meeting Link- ...
Date : 19, Dec 2020
https://orioninfusion-agm-egm.bdvirtual.com... -
NOTICE OF AN EXTRA-ORDINA ...
Date : 5, Dec 2019
ORION INFUSION LIMITED Registered Office: Orion Hou... -
PSI_Press release_Q1_2019 ...
Date : 13, Nov 2019
ORION INFUSION LIMITED Register Office: Orion House, 153-1... -
EGM Notice_OIL ...
Date : 4, Nov 2019
ORION INFUSION LIMITED Registered Office: Orion House, 153... -
Price Sensitive Informati ...
Date : 24, Oct 2019
ORION INFUSION LIMITED Register Office: Orion House, 153-15... -
UN-AUDITED HALF YEARLY (Q ...
Date : 3, Feb 2019
ORION INFUSION LIMITED Register Office: Orion Hou... -
UN-AUDITED 1st QUARTERLY ...
Date : 15, Nov 2018
ORION INFUSION LIMITED Register Office: Orion House... -
UN-AUDITED THIRD QUARTERL ...
Date : 2, May 2017
ORION INFUSION LIMITED Regi... -
Share Bazaar Awareness ...
Date : 18, Feb 2017
দেশব্যাপী বিনিয়োগ শিক্ষা কার্যক্রম বাংলাদেশ সিকিউরিটিজ অ্যা... -
Share Bazaar Awareness ...
Date : 16, Feb 2017
দেশব্যাপী বিনিয়োগ শিক্ষা কার্যক্রমবাংলাদেশ সিকিউরিটিজ অ্যান্... -
UN-AUDITED HALF YEARLY (Q ...
Date : 1, Feb 2017
ORION INFUSION LIMITED Register Office: Orion House, 153-15...
Latest Reports
-
Un-Audited 1st Quarterly Financial Statements
Report of Quarterly Report » 30th September 2016
-
Un-Audited 1st Quarterly Financial Statements 2018
Report of Quarterly Report » 30th September 2018, 1st Quarterly
-
CONDENSED INTERIM FINANCIAL STATEMENTS (UN-AUDITED) AS AT AND FOR SIX MONTH PERIOD ENDED 31ST DECEMBER 2018
Report of Half Yearly Report » 31st December 2018
-
Report of Annual Report » Annual Report 2018
-
FINANCIAL STATEMENTS (UN-AUDITED) FOR THE PERIOD ENDED 31 MARCH 2019 (THIRD QUARTER)
Report of Quarterly Report » 31st March 2019
-
FINANCIAL STATEMENTS (UN-AUDITED) FOR THE PERIOD ENDED 31 MARCH 2018 (THIRD QUARTER)
Report of Quarterly Report » 31st March 2018, 3rd Quarter
-
24 th Annual Report
Report of Annual Report » Annual Report 05-07
-
Statement of Financial Position (Un Audited)
Report of Quarterly Report » 30th September2013
-
3rd Quarter - 2012 - 13 Final
Report of Quarterly Report » 31st March 2013
-
Proxy From-2012
Report of Annual Report » Annual Report 11-12
-
Half Yearly Report
Report of Half Yearly Report » 31st December 2012
-
For the Period of 01 july,2012 to 30 September,2012(1st Quarter)
Report of Quarterly Report » 30th September 2012
-
CONDENSED INTERIM FINANCIAL STATEMENTS (UN-AUDITED) AS AT AND FOR THREE MONTH PERIOD ENDED 30 SEPTEMBER 2019
Report of Quarterly Report » 30th September 2019, 1st Quarter
-
Report of Annual Report » Annual Report 2017
-
FINANCIAL STATEMENTS (UNAUDITED) AS ON 31ST DECEMBER 2017 (HALF YEARLY)
Report of Half Yearly Report » 31st December 2017
-
Financial Statements (Un-Audited) As on 31 March-2016
Report of Quarterly Report » 31st March 2016
-
Financal Statements As On 31 December-2015
Report of Half Yearly Report » 31st December 2015
-
Report of Annual Report » Annual Report 2015
-
31st September-2015 First Quarter
Report of Quarterly Report » 31st September 2015, 1st Quarter
-
Financial Statements(UnAudited)
Report of Quarterly Report » 31st March 2015
-
Finacial Statement31 December-2014 Half Yearly
Report of Half Yearly Report » 31st December-2014
-
Finacial Statement (Un-Audited)As On 30 th September 2016
Report of Quarterly Report » 30th September 2016
-
Report of Annual Report » Annual Report 2016
-
Financial Statements (Un-Addit) As 31 st March-2017
Report of Quarterly Report » 31st March 2017, 3rd Quarter
-
FINANCIAL STATEMENTS (UN-AUDITED) AS ON 30TH SEPTEMBER 2017 (FIRST QUARTER)
Report of Quarterly Report » 30th September 2017, 1st Quarter
-
Financeal Statements (Unaudited)
Report of Quarterly Report » 30th September-2014
-
Annual Report Contents
Report of Annual Report » Annual Report 2014
-
3rd Quarterly Financial Statements (Un Audited) As at 31 march-2014
Report of Quarterly Report » 31st March 2014, 3rd Quarterly
-
Half Year Financlal Statement (Unaudited) As At 31 December-2013
Report of Half Yearly Report » 31st December-2013
-
Infusion Annual Report 2019
Report of Annual Report » Annual Report 2019
-
Report of Half Yearly Report » 31st December 2011
-
Auditor's Report 2009
Report of Annual Report » Annual Report 08-09
-
Auditor's Report 1990
Report of Annual Report » Audit Report 1990-2004
-
Report of Annual Report » Annual Report 2021
-
FINANCIAL STATEMENTS (UNAUDITED) AS ON 31ST DECEMBER 2016 (HALF YEARLY)
Report of Half Yearly Report » 31st December 2016
-
Half yearly 2010
Report of Half Yearly Report » 31st December 2010
-
31 March2011 3rd Quarter
Report of Quarterly Report » 31st March 2011
-
ANNUAL REPORT 2010-2011
Report of Annual Report » Annual Report 10-11
-
Report of Quarterly Report » SEPTEMBER 30, 2023 (FIRST QUARTER)
-
Annual Report 2009-2010
Report of Annual Report » Annual Report 09-10
-
Un-Audited Half Yearly (Q2) Financial Statements
Report of Half Yearly Report » Half Yearly Report 2023
-
FINANCIAL STATEMENTS (UN-AUDITED) FOR THE PERIOD ENDED 31 MARCH 2019 (THIRD QUARTER)
Report of Half Yearly Report » 31st December 2019, 2020, 2021
-
Auditor's Report 2020
Report of Annual Report » Annual Report 2020
-
AS AT AND FOR NINE MONTH PERIOD ENDED 31 MARCH 2020 ORION INFUSION LIMITED (THIRD QUARTER)
Report of Quarterly Report » 30th March 2020, 3rd Quarter
-
FINANCIAL POSITION (UN-AUDITED) AS AT SEPTEMBER 30, 2021
Report of Quarterly Report » 30th September 2021
-
CONDENSED INTERIM FINANCIAL STATEMENTS (UN-AUDITED) AS AT AND FOR THREE MONTH PERIOD ENDED 30 SEPTEMBER 2020 ORION INFUSION LIMITED (FIRST QUARTER)
Report of Quarterly Report » 30th September 2020
-
General Information
Report of Annual Report » Annual Report 10-11
-
29th Annual Report-2012
Report of Annual Report » Annual Report 11-12
-
Board Of Directors
Report of Annual Report » Annual Report 2014
-
Auditor's Report 1991
Report of Annual Report » Audit Report 1990-2004
-
Director's Report 2020
Report of Annual Report » Annual Report 2020
-
Financal Statements As On 31-December-2023
Report of Half Yearly Report » Half Yearly Report 2023
-
Infusion Annual Report-2019 page19-36
Report of Annual Report » Annual Report 2019
-
CONDENSED INTERIM FINANCIAL STATEMENTS (UN-AUDITED)AS AT AND FOR NINE MONTH PERIOD ENDED MARCH 31, 2021
Report of Quarterly Report » 30th March 2020, 3rd Quarter
-
FINANCIAL STATEMENTS (UN-AUDITED) FOR THE PERIOD ENDED DECEMBER 31, 2020 (HALF YEARLY)
Report of Half Yearly Report » 31st December 2019, 2020, 2021
-
Our Vision Mission & Values
Report of Annual Report » Annual Report 09-10
-
Financial Report 2006-2007
Report of Annual Report » Annual Report 05-07
-
Message From the Chairman
Report of Annual Report » Annual Report 07-08
-
SIX MONTH PERIOD ENDED DECEMBER 31, 2021 (HALF YEARLY)
Report of Half Yearly Report » 31st December 2019, 2020, 2021
-
Board of Directors
Report of Annual Report » Annual Report 10-11
-
Infusion Annual Report-2019 page37-72
Report of Annual Report » Annual Report 2019
-
Corporate Social Responsibility(CSR)
Report of Annual Report » Annual Report 2014
-
Board of Directors
Report of Annual Report » Annual Report 09-10
-
Auditor's Report 1992
Report of Annual Report » Audit Report 1990-2004
-
Quality Policy
Report of Annual Report » Annual Report 05-07
-
25 Annual Report
Report of Annual Report » Annual Report 07-08
-
Auditor's Report 2007
Report of Annual Report » Annual Report 05-07
-
Auditor's Report 2008
Report of Annual Report » Annual Report 07-08
-
Infusion Annual Report-2019 page73-90
Report of Annual Report » Annual Report 2019
-
Auditor's Report 1993
Report of Annual Report » Audit Report 1990-2004
-
CC
Report of Quarterly Report » 30th September 2021
-
Management Committee
Report of Annual Report » Annual Report 09-10
-
AGM-2013 Proxy Form
Report of Annual Report » Annual Report 2013
-
Annual General Meeating-2013
Report of Annual Report » Annual Report 2014
-
Chairman Profile
Report of Annual Report » Annual Report 10-11
-
Company at a Glance
Report of Annual Report » Annual Report 09-10
-
Infusion Annual Report-2019 page91-108
Report of Annual Report » Annual Report 2019
-
Auditor's Report 1994
Report of Annual Report » Audit Report 1990-2004
-
Company Secretary & Management Committee
Report of Annual Report » Annual Report 10-11
-
Auditor's Report 2006
Report of Annual Report » Annual Report 05-07
-
Annexure To The director's Report
Report of Annual Report » Annual Report 2014
-
Infusion Annual Report-2019 page109-127
Report of Annual Report » Annual Report 2019
-
Auditor's Report 2005
Report of Annual Report » Annual Report 05-07
-
Quality Policy & ISO 9001:2000
Report of Annual Report » Annual Report 09-10
-
Annexure To The director's Report
Report of Annual Report » Annual Report 2014
-
Auditor's Report 1995
Report of Annual Report » Audit Report 1990-2004
-
Notes To The Financial Statements
Report of Annual Report » Annual Report 2014
-
Auditor's Report 1996
Report of Annual Report » Audit Report 1990-2004
-
Human Resource Development
Report of Annual Report » Annual Report 09-10
-
Company at a Glance
Report of Annual Report » Annual Report 10-11
-
Annual General Meeating-2013-14
Report of Annual Report » Annual Report 2014
-
Auditor's Report 1997
Report of Annual Report » Audit Report 1990-2004
-
Strategic Business Units
Report of Annual Report » Annual Report 10-11
-
Annul General Meeting-2009
Report of Annual Report » Annual Report 09-10
-
Product List
Report of Annual Report » Annual Report 09-10
-
Our Vision, Our Mission Goals & Values
Report of Annual Report » Annual Report 10-11
-
Company History
Report of Annual Report » Annual Report 10-11
-
5 Years At A Glance
Report of Annual Report » Annual Report 09-10
-
Financial Report 2012-2013
Report of Annual Report » Annual Report 2013
-
Message From The Chairman
Report of Annual Report » Annual Report 09-10
-
Financial Report 2012-2013
Report of Annual Report » Annual Report 2013
-
Quality Policy
Report of Annual Report » Annual Report 10-11
-
Financial Report 2009-2010
Report of Annual Report » Annual Report 09-10
-
Financial Report 2012-2013
Report of Annual Report » Annual Report 2013
-
Human Resources Development
Report of Annual Report » Annual Report 10-11
-
Brand Performance
Report of Annual Report » Annual Report 10-11
-
Financial Report 2009-2010 & Proxy From
Report of Annual Report » Annual Report 09-10
-
Financial Report 2012-2013
Report of Annual Report » Annual Report 2013
-
Annual General Meeting 2010 & Annual Marketing & Sales Conference
Report of Annual Report » Annual Report 10-11
-
Company at a Glance
Report of Annual Report » Annual Report 2013
-
Auditor's Report 1998
Report of Annual Report » Audit Report 1990-2004
-
Product List
Report of Annual Report » Annual Report 10-11
-
Quality Policy
Report of Annual Report » Annual Report 2013
-
Auditor's Report 1999
Report of Annual Report » Audit Report 1990-2004
-
5 Years At A Glance
Report of Annual Report » Annual Report 10-11
-
Financial Report 2010-2011
Report of Annual Report » Annual Report 10-11
-
Message From the Chairman
Report of Annual Report » Annual Report 2013
-
Auditor's Report 2000
Report of Annual Report » Audit Report 1990-2004
-
Annexure To the Directors\' Report
Report of Annual Report » Annual Report 2013
-
Notes of the Financial Statements For The year Ended June30,2011
Report of Annual Report » Annual Report 10-11
-
Auditor's Report 2001
Report of Annual Report » Audit Report 1990-2004
-
Message From The Chairman
Report of Annual Report » Annual Report 10-11
-
Auditor's Report 2002
Report of Annual Report » Audit Report 1990-2004
-
Auditor's Report 2003
Report of Annual Report » Audit Report 1990-2004
-
Directors’ Report To the Shareholders
Report of Annual Report » Annual Report 10-11
-
Auditor's Report 2004
Report of Annual Report » Audit Report 1990-2004
Plasmasol
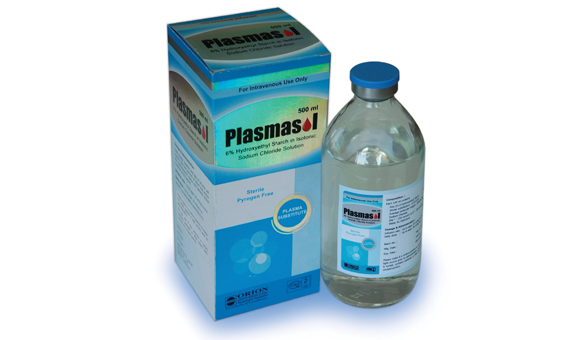
Plasmasol
Description :
PLASMASOL is a sterile solution containing 6% Hydroxyethyl Starch (200/0.5) in isotonic Sodium Chloride solution. It is a clear, pale yellow solution.
Composition :
Each 100 ml contains: Poly (O-2-hydroxyethyl) starch INN 6.0 g, Sodium Chloride BP 0.9 g (Equals 154 mmol/L Na+ and 154 mmol/L Cl-), water for Injections BP q.s to 100 ml, Osmolarity (Theoretical) 308 mOsm/L, pH 3.5-6.0;
Indications :
PLASMASOL infusion is indicated in the treatment of hypovolaemia when plasma volume expansion is desired as an adjunct in the management of shock due to haemorrhage, surgery, sepsis, burns or other trauma. It is not a substitute for red blood cells or coagulation factors in plasma. The adjunctive use of PLASMASOL infusion in leukapheresis has also been shown to be safe and efficacious in improving the harvesting and increasing the yield of granulocytes by centrifugal means.
Dosage and administration :
PLASMASOL must be administered intravenously. Total dosage, duration and rate of infusion will depend upon the amount of blood lost and/or the haemodynamic status and general clinical condition of the patient. Dosage will need to be adjusted as necessary by monitoring the usual circulatory parameters e.g. blood pressure. The risk of circulatory overload by too rapid rate of infusion or inappropriately large doses must be borne in mind. Due to the risk for occurrence of an anaphylactic reaction, the first 10 ml - 20 ml of PLASMASOL should be infused slowly under careful observation of the patient. Maximum infusion rate: The maximum rate of infusion should be adjusted according to the clinical situation. Patients with acute haemorrhagic shock: Up to 20 ml/kg body weight/hour. In life-threatening situations: 500 ml as a rapid infusion (under pressure). The rates of infusion selected for perioperative indications and for burns and septic shock patients will usually be lowered. Maximum daily dosage : A maximum daily dosage of 2 g/kg body weight/day of Hydroxyethyl starch should not be exceeded. This corresponds to 33 ml/kg body weight/day of 6% Hydroxyethyl starch solution (approximately 2,500 ml/day in a person of 75 kg). Experience of treatment of more than 1-2 days is limited. In cases of longer treatment the daily doses have generally been lower. An increasing risk of undesirable effects with high cumulative doses should be considered. Dosage in Leukapheresis: 250 to 700 ml of PLASMASOL infusion to which citrate anticoagulant has been added is typically administered by aseptic addition to the input line of the centrifugation apparatus at a ratio of 1:8 to 1:13 to venous whole blood. The PLASMASOL infusion and citrate should be thoroughly mixed to assure effective anticoagulation of blood as it flows through the leukapheresis machine.
Use in children:
The safety and efficacy of PLASMASOL infusion in children has not been established. Administration to children should only be managed after careful benefit-risk assessment.
Use in pregnancy :
Animal reproduction studies have not been conducted with PLASMASOL infusion and it is also not known whether it can cause fetal harm or affect reproductive capacity when administered during pregnancy. PLASMASOL infusion should be given during pregnancy only if clearly needed
Use during lactation :
It is not known whether Hydroxyethyl starch is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PLASMASOL infusion is administered to a nursing woman. Contraindications: Known hypersensitivity to Hydroxyethyl starch, hypervolaemia, hyper-hydration (e.g. water intoxication), hyperchloraemia (or hypernatraemia), congestive cardiac failure, pulmonary oedema, renal failure with oliguria and anuria not related to hypervolaemia, cerebral haemorrhage, severe blood coagulation disorders & severe hepatic impairment.
Adverse effects :
The following adverse effects have been reported: vomiting, fever, chills, pruritus, submaxillary and parotid glandular enlargement, mild influenza-like symptoms, headaches, muscle pains, peripheral edema of the lower extremities, anaphylactoid reactions (periorbital edema, urticaria, wheezing), bleeding due to haemodilution, circulatory overload and pulmonary edema.
Interactions : The safety and compatibility of other additives, other than citrate anticoagulant, have not been established. Incompatibilities: In the absence of incompatibility studies this medicinal product must not be mixed with other medicinal products.
Caution : Dextrosal is available in 500ml & 1000ml PVC (medical grade) bag.
Storage condition : Store in a dry and cool place protected against intensive light, at a temperature not exceeding 25 °C. Keep out of reach of children.
Special precautions for storage : Do not freeze.
Pack size: PLASMASOL is available in 500 ml glass bottle.